The study of molecular compounds for celestial bodies as galaxies, stars, and the planets helps human gains more insights on physical conditions of the Universe, such as the temperature and density. The astrochemistry enables man to find out how molecules in space relate to life inside the earth planet. The intellectuality of astrochemistry research leads to the development of technologies that are vital for human lives.
Definition
Astrochemistry is the chemistry of astronomical objects/bodies, such as galaxies, stars and planet, in which molecular compounds exist. It embraces observations of molecular absorption and emission spectra and their use as diagnostic probes of the physical environment and the study of the influence of molecular composition on the behavior of the objects.
Astrochemistry can also be define “as the study of molecules in the universe; its studies how molecules are formed, destroyed, how complex they can get, how does the molecular composition vary from place to place, use them (molecules) as a tracer of physical conditions (temperature, density), and how do molecules in space relate to life as we know it”.
Basic to astrochemistry are the elementary processes of molecular formation in the gas phase and on the surfaces of solid particles and the response of molecules to electromagnetic and corpuscular radiations and to dynamical interactions. Astrochemistry draws its inspiration, language, fascination, beauty, elegance and confusion from many different disciplines; starting with astronomy, passing through physical chemistry and ending with the new ideas of astrobiology.
NOTE: A molecule is a collection of positively charged atomic nuclei surrounded by a cloud of negatively charged electrons. Its stability results from a balance among the attractive and repulsive forces of the nuclei and electrons. A molecule is characterized by the total energy resulting from these interacting forces. As is the case with atoms, the allowed energy states of a molecule are quantized.
Overview of Astrochemistry
Astrochemistry spans the disciplines of chemistry, planetary science, chemical biology, physics, astronomy, and computational science. Astrochemists perform experimental and computational laboratory studies to generate data for interpreting or explaining astronomical observations, to provide input data for models, and to test theories about the formation and evolution of large and small molecules in various astrophysical environments.
Astrochemists use Earth-based telescopes, satellites, and space vehicles to gather spectroscopic data. They create and apply mathematical models, theories based on chemical dynamics, kinetics, quantum mechanics, and other physical principles. They use computer visualizations to assist them explain their observations in terms of known physical and chemical principles and to study the origins of extraterrestrial bodies and the chemical processes that have shaped their present forms.
Also, Astrochemists examine chemical compositions and processes for stars, planets, comets, and interstellar media. They look at how atoms, molecules, ions, and free radicals interact outside of Earth's atmosphere. They contribute to understanding of geological processes on other planets, and they explore conditions under which life might form by examining molecules on other planets and in outer space.
The Process of Detecting Presence of Molecules in Galaxies and Other Planets
Astronomers identify interstellar atoms and molecules through spectroscopy. For example, interstellar sodium atoms that happen to be in a line of sight going from a point on Earth's surface toward a bright star, absorb light emitted by that star at a wavelength that is about (589 nanometers: 2.3 × 10−5 inches). Most interstellar molecules are detected by spectroscopic analysis that measures absorption or emission at radio wavelengths rather than those corresponding to visual light. Astronomers use large radio telescopes to detect radiation emitted by interstellar molecules. These emissions arise because the molecules are set to rotating when they collide with each other. The molecules lose energy and slowdown in their rotations by emitting radiation at wavelengths that are specific for them, such that each emission is a signature of one type of molecule. For instance, the molecule carbon monoxide, CO, may emit at various radio wavelengths, including 2.6 millimeters (0.1 inches), 1.3 millimeters (0.05 inches), 0.65 millimeters (0.03 inches), and 0.32 millimeters (0.01 inches). Interstellar gas is usually very cold, but even under these coolest conditions, the molecular collisions are energetic enough to keep the molecules rotating and, therefore, emitting radiation.
Sometimes, these interstellar molecules may be located in warmer regions. If the gas of which they are a part is close to a star, or becomes heated because one clump collides with another, the temperature of the molecules may rise considerably, perhaps to several thousand degrees above absolute zero. In these cases, the collisions between gas molecules are more energetic, and molecules may be set to vibrating as well as rotating. For example, a carbon monoxide molecule vibrates to-and-fro as if the two atoms are connected by a coiled spring. A vibrating molecule also eventually slows down and loses energy (unless it is involved in further collisions) by emitting radiation that is again specific to that particular molecule. In the example of carbon monoxide molecule CO, that radiation has a wavelength of about 4.7 micrometers (18.5 × 10−5 inches), the detection of which necessitates the use of large telescopes that are sensitive to infrared radiation.
Formation of Molecules at Interstellar
The Milky Way, like all other galaxies, was formed from intergalactic gas that was essentially atomic. So where do the molecules come from? One can deduce that they are not left over from the processes that formed the Milky Way, because scientists can detect molecules in regions in which they are being rapidly destroyed, therefore there must be a formation process in operation now. For example, the hydroxyl molecule, OH, can be observed in rather low-density interstellar gas regions in which it is being destroyed by stellar radiation in a time frame, typically, of ten thousand years. This seems a long time but because the Galaxy has been in existence for a much longer time - approximately 15 billion years, the OH radicals must have been formed relatively recently in the Galaxy's history.
Simple collisions between O and H atoms do not lead to the formation of OH molecules, because the atoms bounce apart before they are able to form a chemical bond. Similarly, low temperature collisions between O atoms and H2 molecules are also unreactive. So, how does the Interstellar molecules are being formed?
Astronomers and chemists have now determined that much of the chemistry of interstellar space occurs via ion-molecule reactions. Cosmic rays ionize molecular hydrogen H2 and the resulting ions (H2+) react quickly with more H2 to form other ions (H3+). The H3+ ions drive a chemistry that consists of simple two-body reactions. The extra proton in H3+ is quite weakly bound (relative to the bonding of one proton to another in H2). In a collision, an H3+ molecule easily donates its proton to some other species, creating a new molecule. For example, an H3+ ion reacts with an O atom to give OH+, a new species:
O + H3+ → OH+ + H
and the OH+ then reacts with H2 molecules to make, successively, H2O+ and H3O+ ions:
OH+ → H2O+ → H3O+
This process of H abstraction finishes here, because the O+ ion in H3O+ has saturated all its valence’s with respect to H atoms. However, the H3O+ ion has a strong attraction for electrons because of its positive charge, and the ion-electron recombination leads to dissociation of the ion-electron complex into a variety of products, including OH (hydroxyl) and H2O (water).
Other exchange reactions occur, for example, CO may be formed through the neutral exchange:
C + OH → CO + H.
Similar ion-molecule reactions drive the chemistry of other atoms, such as C and N, to yield ions such as CH3+ and NH3+. These ions can then react with other species to form larger and more complex molecules. For example, methanol (CH3OH) may be formed by the reaction of CH3+ ions with H2O molecules, followed by recombination of the product of that reaction with electrons:
CH3+ + H2O → CH3OH2+
CH3 OH2+ + e− → CH3 OH + H
Ion-molecule reactions, followed by ion-electron recombination and supplemented by neutral exchanges, are capable of forming the majority of the observed interstellar molecular species. Very large gas-phase reaction networks, involving some hundreds of species interacting in some thousands of chemical reactions, are routinely used to describe the formation of the observed interstellar molecules in different locations in models of interstellar chemistry.
Role of Dust in Astrochemistry
The dust has several important chemical roles. Obviously, it may shield molecules from the destructive effects of stellar radiation. It also has more active roles. It has been seen that free atoms in collision may simply bounce apart before they can form a chemical bond. By contrast, atoms adsorbed on the surface of a dust grain may be held together until reaction occurs. It’s also believed that molecular hydrogen is formed in this way, i.e. through heterogeneous catalysis, and is ejected from dust grain surfaces into the gas volume with high speed and in high states of vibration and rotation. Other simple molecules, such as H2O, CH4, and NH3, are also likely to form in this way.
In the denser clumps where the gas is very cold, the dust grains are also at a very low temperature. Gas-phase molecules colliding with such grains tend to stick to their surfaces, and over a period of time, the grains in these regions accumulate mantles of ice, mostly H2O ice, but also ices containing other molecules such as CO, CO2, and CH3OH. Astronomers can detect these ices with spectroscopy. For example, water ice molecules absorb radiation at a wavelength about 3.0 micrometers (11.8 × 10−5 inches), having to do with the O–H vibration in H2O molecules, the molecules do not rotate because they are locked into the ice. In instances in which such ice-coated dust grains lie along a line of sight toward a star that shines in the infrared, this 3.0 micrometer absorption is very commonly seen.
Interstellar solid-state chemistry can occur within these ices. Laboratory experiments have shown that ices of simple species such as H2O, CO, or NH3 can be stimulated by ultraviolet radiation or fast particles (protons, electrons) to form complex molecules, including polycyclic aromatic hydrocarbons (PAHs) containing several benzene-type rings. The detection by astronomers of free interstellar benzene (C6H6) in at least one interstellar region suggests that this solid-state chemistry might be the route by which these molecules are made.
Roles of molecules in Astronomy
The primary role that interstellar molecules play is a passive one! Their presence in regions so obscured by dust that we cannot see into them using optical telescopes is used to probe these regions. The most dramatic example of this is the discovery of the so-called giant molecular clouds in the Milky Way and other galaxies through the detection of the emission of 2.6 micrometers (10.2 × 10−5 inches) wavelength radiation by carbon monoxide (CO) molecules present in these clouds. The existence of these huge gas clouds, containing up to a million times the mass of the Sun - MSun, was not suspected from optical observations because these clouds are completely shrouded in dust. However, radio astronomy has shown that these clouds are the largest non-stellar structures in the Galaxy, and that they will provide the raw material for the formation of millions of new stars in future billions of years of the Galaxy's evolution.
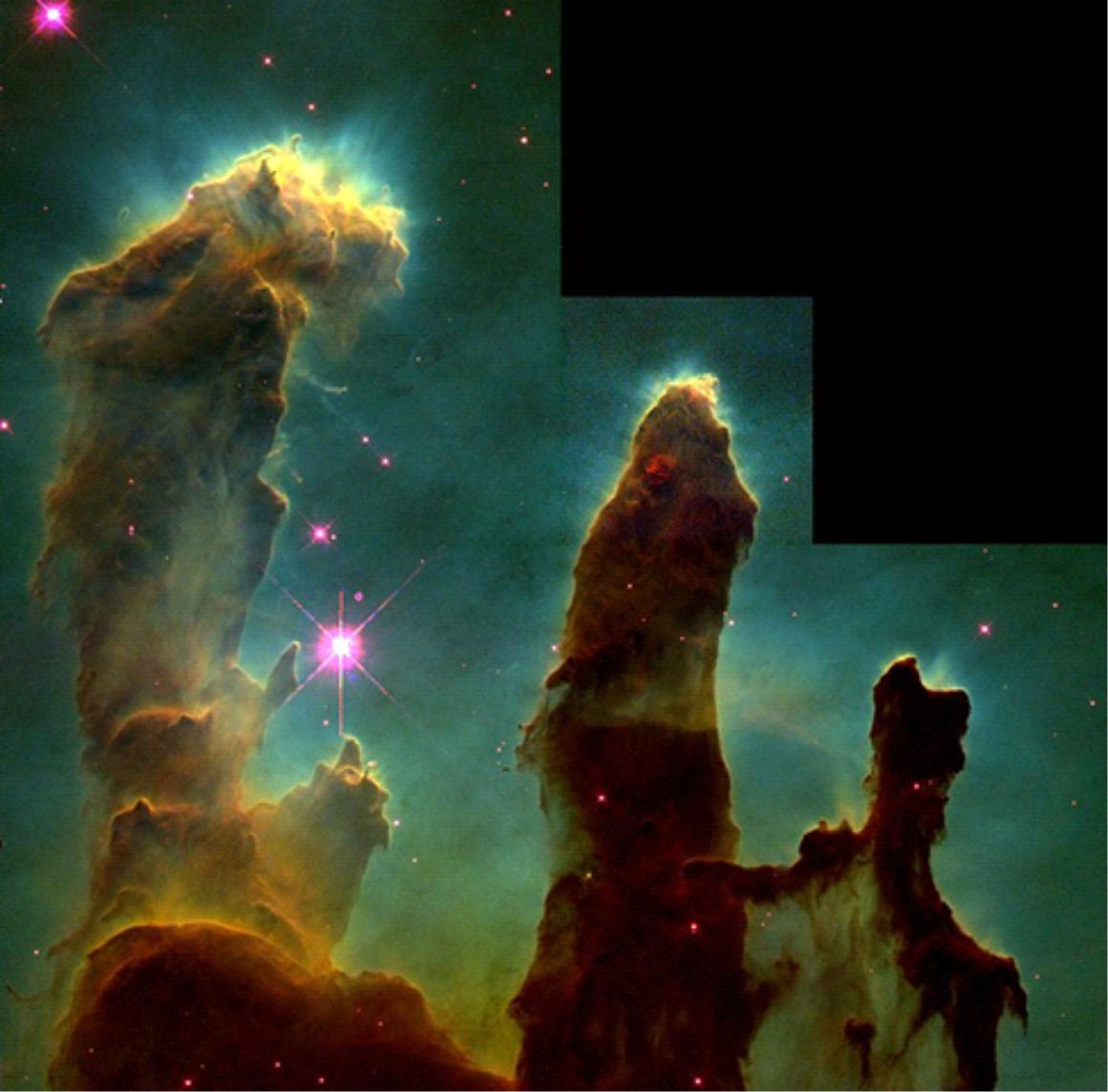
The radiation from molecules that had been detected can represent a significant loss of energy from an interstellar cloud. Some molecules are very effective coolants of interstellar gases and help to maintain the temperatures of these gases at very low values. This cooling property is very important in clumps of gas that are collapsing inward under their own weight. If such a collapse can continue over vast stretches of time, then ultimately a star will form. In the early stages, it is important that the clumps remain cool, otherwise the gas pressure might halt the collapse. In these stages, therefore, the cooling effect of the molecules' emission of radiation is crucial. The formation of stars like the Sun is possible because of the cooling effect of molecules. Interstellar chemistry is therefore one factor determining the rate of star formation in the Galaxy. Astrochemists have shown that it takes about one million years for the molecules of a collapsing cloud to be formed! This is about the same amount of time as that required for the collapse itself to become established. The accompanying image illustrates a region of star formation in the Galaxy.
A star-forming region: pillars of gas in the Eagle Nebula (M16). A molecular cloud is eroded by the winds and radiation of nearby very bright stars (not shown, top right in figure), leaving these columns of denser and more resistant gas. Star formation is occurring in very dense globules of gas, some of which can be seen around the periphery of the pillars.
Image credit: New view of the Pillars of Creation — visible, NASA, ESA/Hubble and the Hubble Heritage Team. Retrieved from: https://www.spacetelescope.org/images/heic1501a/ on 22 March 2020.
Astrochemistry also has a role that is particularly significant to the human species here on planet Earth. The planet was believed by scientist formed as a byproduct of the formation of the star that is the Sun, and is in effect the accumulation of dust grains that were the debris of large chunks of matter that subsequently impacted and stuck together. Earth is still subject to the occasional impacts of debris left over from the formation of the solar system. These impacts, now seen as a source of potential danger, in fact once brought prebiotic material to Earth. The oceans arose from the arrival of icy comets, and carbon, nitrogen, and elemental metals were brought by asteroid impacts. These elements and others are necessary for life on Earth.
SPECTROSCOPY
One particularly important experimental tool in astrochemistry is spectroscopy, through the use of telescopes to measure the absorption and emission of light from molecules and atoms in various environments. By comparing astronomical observations with laboratory measurements, astrochemists can infer the elemental abundances, chemical composition, and temperatures of stars and interstellar clouds. This is possible because ions, atoms, and molecules have characteristic spectra - that is, the absorption and emission of certain wavelengths (colors) of light, often not visible to the human eye.
Spectroscopy therefore, is the study of the absorption and emission of light and other radiation by matter, as related to the dependence of these processes on the wavelength of the radiation. More recently, the definition has been expanded to include the study of the interactions between particles such as electrons, protons, and ions, as well as their interaction with other particles as a function of their collision energy.
History of spectroscopy
The basis for analytical spectroscopy is the discovery, made in 1859 by the German physicist Gustav R. Kirchhoff, that each pure substance has its own characteristic spectrum. Another German physicist, Joseph von Fraunhofer, repeating more carefully an earlier experiment by a British scientist, William Wollaston, had shown in 1814 that the spectrum of the Sun’s electromagnetic radiation does not grade smoothly from one colour to the next but has many dark lines, indicating that light is missing at certain wavelengths because of absorption. These dark lines, sometimes called Fraunhofer lines, are also collectively referred to as an absorption spectrum. The spectra of materials that were heated in flames or placed in electric-gas discharges were studied by many scientists during the 18th and 19th centuries. These spectra were composed of numerous bright discrete lines, indicating that only certain wavelengths were present in the emitted light. They are called brightline, or emission, spectra.
Although the possibility that each chemical element has a unique characteristic spectrum had been considered by numerous investigators, the early studies were hindered by the difficulty of obtaining relatively pure substances. Any sample could contain impurities that would result in the simultaneous production of many spectra. By using carefully purified substances, Kirchhoff demonstrated characteristic spectra and initiated the technique of spectroscopic analysis of the chemical composition of matter. The technique was applied by Kirchhoff and his colleague the German chemist Robert Bunsen in 1861 to the analysis of the Sun’s electromagnetic spectrum and the identification of the chemical elements in the Sun.
Before the 20th century, there was no theory that could satisfactorily explain the origin of the spectra of the elements or the reason why different elements have different spectra. The quantitative understanding of the elemental spectra needed the development of a fundamentally new physical theory, and the spectra of the simplest atoms played the key role in the development of this theory. Many of the major developments in 20th-century physics were motivated by an ever-increasing accuracy in the measurement of the spectra of the hydrogen atom; highlights include the discovery in 1885 by the Swiss scientist Johann J. Balmer that the frequency spectrum of hydrogen followed a simple numerical pattern, later revised by the Swedish physicist Johannes R. Rydberg and given in modern notation as 1/λ = RH (1/22 − 1/n2), where RH is the so-called Rydberg constant for hydrogen. In 1913, the Danish physicist Niels Bohr presented the first theoretical model that could give quantized energy levels that were in quantitative agreement with measurements of the hydrogen spectrum.
Despite the success of the Bohr theory in describing the hydrogen spectrum, the theory failed badly when applied to the next simplest atom, helium, which contains two electrons. It was also incapable of predicting the likelihood of transitions between energy levels. In 1925–26 a new theory that could explain the discrete, quantum nature of the spectra was developed by the German physicists Werner Heisenberg and Erwin Schrödinger. This theory, known as quantum mechanics, was extended by the Austrian-born Swiss physicist Wolfgang Pauli, the German physicist Max Born, and others. It has been remarkably successful in describing the spectra of complex atoms, ions, simple molecules, and solids.
As the spectral lines of the hydrogen atom were measured with increased accuracy, greater demands were placed on the theoretical understanding of atomic spectra. The British physicist Paul A.M. Dirac combined quantum mechanics with the special theory of relativity in 1928 to describe particles moving close to the speed of light. His formulation of relativistic quantum mechanics provided an explanation for the so-called fine structure of the hydrogen spectrum. At still higher resolution, two energy levels of the hydrogen atom in the first excited state were predicted by Dirac’s theory to be exactly the same. In 1947, the American physicists Willis Lamb and Robert Retherford discovered that the levels actually differ by roughly 109 hertz. In contrast, the transition frequency between the ground state and the first excited states was calculated as approximately 2.5 × 1015hertz. Two American physicists, Richard Feynman and Julian Schwinger, and a Japanese physicist, Shinichirō Tomonaga, developed yet another refinement to quantum mechanics to explain this measurement. The theory, known as quantum electrodynamics (QED), had its foundations in the discoveries of Dirac, Heisenberg, and Pauli. It is a complete description of the interaction of radiation with matter and has been used to calculate the energy levels of the hydrogen atom to an accuracy of better than 1 part in 1011. No other physical theory has the ability to predict a measurable quantity with such precision, and, as a result of the successes of quantum electrodynamics, the theory has become the paradigm of physical theories at the microscopic level.
Applications of Spectroscopy
Spectroscopic techniques have been applied in virtually all technical fields of science and technology. Radio-frequency spectroscopy of nuclei in a magnetic field has been employed in a medical technique called magnetic resonance imaging (MRI) to visualize the internal soft tissue of the body with unprecedented resolution.
Microwave spectroscopy was used to discover the so-called three-degree blackbody radiation, the remnant of the big bang (i.e., the primeval explosion) from which the universe is thought to have originated. The internal structure of the proton and neutron and the state of the early universe up to the first thousandth of a second of its existence are being unraveled with spectroscopic techniques using high-energy particle accelerators. The constituents of distant stars, intergalactic molecules, and even the primordial abundance of the elements can be determined by optical, radio, and X-ray spectroscopy. Optical spectroscopy is used routinely to identify the chemical composition of matter and to determine its physical structure.
The astrochemistry is an intellectually exciting area of research which remains vital into the foreseeable future. It embraces many disciplines and enhances them by the mutual interactions that it stimulates. Astrochemistry addresses deeply significant issues arising in the formation of galaxies, stars and the planets, and ultimately perhaps the origin of life. The unification of early Universe chemistry, interstellar chemistry, circumstellar and stellar chemistry, cometary and meteoritic chemistry (together with planetary atmosphere chemistry) is a challenging task which requires research in many different branches of Physics, Chemistry, Geology and Astronomy. Its successful completion will teach us much more about the Universe and its evolution.
REFERENCES
- Dalgarno, A. (1985). Astrochemistry - a Summary: Journal of Astrochemistry, Proceedings of the IAU Symposium #120. Tarafdar. Dordrecht, D. Reidel Publishing Co., p. 577, 1987. Retrieved from:http://articles.adsabs.harvard.edu//full/1987IAUS..120..577D/0000577.000.html on 10th of March, 2020.
- David A. Williams. Astrochemistry: Chemistry Explained. Retrieved from: http://www.chemistryexplained.com/Ar-Bo/Astrochemistry.html on 10th of March, 2020.
- Astrochemistry: Wikipedia. Retrieved from: https://en.wikipedia.org/wiki/Astrochemistry on 8th of March, 2020.
- John O. S., Jack D. G. and others (2018). Spectroscopy, Encyclopaedia Britannica. Retrieved from: https://www.britannica.com/science/spectroscopy on 8th of March, 2020.
 Bluehole Techs
Bluehole Techs

No comments:
Post a Comment